When you pick up a prescription labeled as a generic, you might assume it’s made by a different company - maybe one you’ve never heard of, somewhere overseas. But what if that same pill, in the exact same bottle, was made by the very same company that invented the brand-name drug? That’s the reality of authorized generics.
What Exactly Is an Authorized Generic?
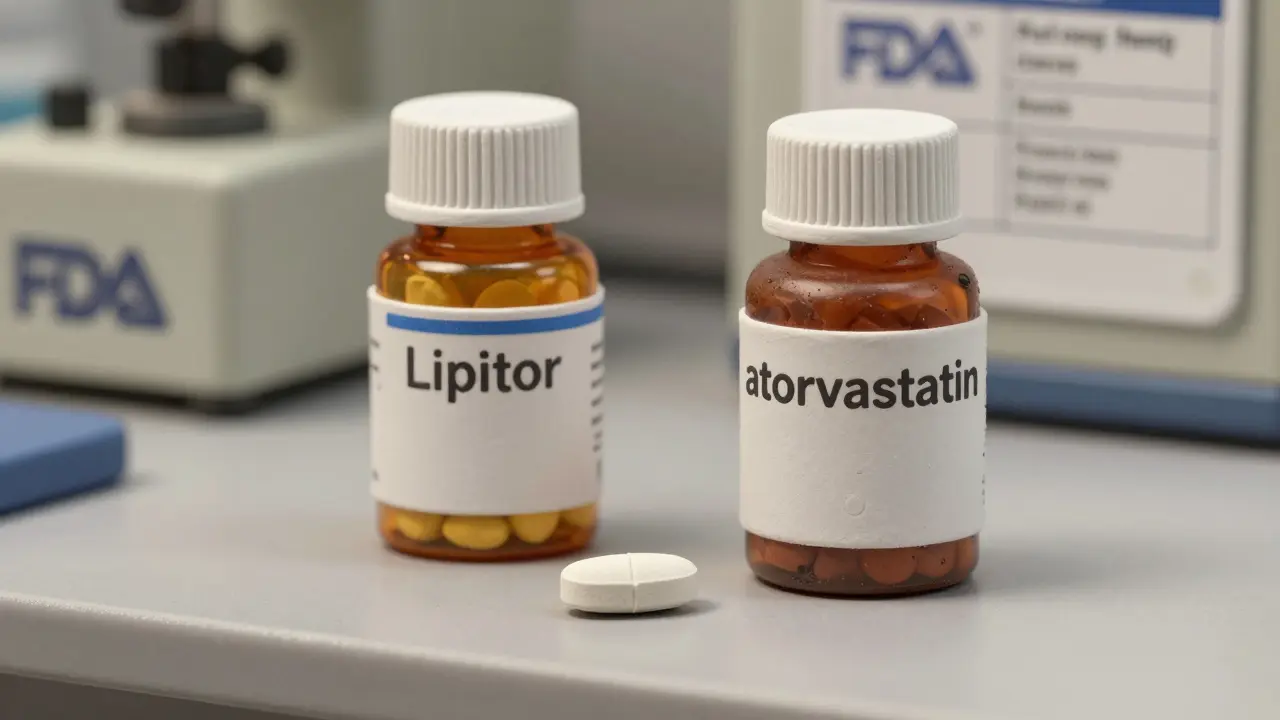
An authorized generic isn’t a copy. It’s the real thing - same active ingredient, same manufacturing process, same factory, same quality control. The only difference? The label. Instead of showing the brand name like "Lipitor" or "Nexium," it says something plain like "atorvastatin" or "esomeprazole." The FDA defines it clearly: it’s the original brand-name drug, approved under the same New Drug Application (NDA), but sold under a private label at generic prices.
This isn’t a loophole. It’s a legal pathway created by the Hatch-Waxman Act of 1984. The law was meant to speed up generic drug approvals, but it also let brand companies protect their market share by launching their own cheaper versions - before their patents even expired. That’s why you’ll sometimes see an authorized generic hit the shelves months before any other generic even gets approved.
Who Makes These Drugs? It’s Not Who You Think
The biggest myth about authorized generics is that they’re made by third-party generic manufacturers like Teva or Mylan. In reality, the majority are made by the original brand company - or a company they own.
Take Pfizer. Their subsidiary, Greenstone LLC, has been making authorized generics since 1998. Greenstone doesn’t just make copies - it makes the exact same pills as Pfizer’s branded drugs, using the same machines, the same chemists, the same quality tests. Over 70 authorized generics come out of Greenstone’s facilities, including versions of drugs like Lyrica and Zoloft. The only thing that changes is the box and the label.
According to FDA data from Q3 2023, about 52% of authorized generics are manufactured directly by the brand-name company. Another 31% are made by wholly-owned subsidiaries like Greenstone. That means more than 80% of authorized generics are made under the same roof as the brand-name version.
Only 17% are outsourced to third-party manufacturers. Even then, the brand company still controls everything: the formula, the sourcing of raw materials, the testing protocols. The FDA requires the NDA holder to maintain full regulatory responsibility - no matter who physically touches the pill.
Same Factory. Same Process. Same Quality.
Here’s the key difference between authorized generics and regular generics: where they’re made.
Regular generics? About 88% are made in completely separate facilities - often in India, China, or other countries with lower labor costs. The formulation might be identical on paper, but the manufacturing environment, equipment, and even the batch-to-batch consistency can vary.
Authorized generics? Around 68% are made in the exact same facility as the brand-name drug. The same filling lines. The same packaging machines. The same inspectors. The same quality control logs. That’s why the FDA’s 2022 inspection data showed a 98.7% compliance rate for authorized generic facilities - higher than the 96.2% for traditional generic plants.
Take the case of AstraZeneca’s Nexium. When they launched their authorized generic version through their subsidiary Az generici, they didn’t change a single step in the manufacturing process. Same granulation. Same drying time. Same tablet hardness. The only thing different? The label said "esomeprazole magnesium" instead of "Nexium." And it sold for 80% less - while still being the exact same drug.

Why Do Brand Companies Do This?
It’s not charity. It’s strategy.
When a brand drug’s patent is about to expire, generic competitors start lining up. The moment the patent falls, dozens of companies can rush in with cheaper versions. Prices crash - sometimes by 90% within months.
By launching their own authorized generic, the brand company gets a head start. They capture the generic market before anyone else can. They keep control over supply. They avoid the chaos of multiple manufacturers with inconsistent quality. And they still make money - just at a lower price point.
Some experts call this a smart business move. Others call it a tactic to delay true competition. Dr. Aaron Kesselheim from Harvard Medical School argues that authorized generics can actually keep prices higher by blocking independent generics from gaining market share. But the FDA’s stance is clear: if the drug meets the same standards, it’s still a win for patients who need affordable access.
The Regulatory Tightrope
Manufacturing an authorized generic isn’t as simple as slapping a new label on a bottle. The FDA requires strict oversight.
If the brand company wants to make it themselves, they just need to file an annual report and update the label. Easy. But if they outsource to another company - say, a contract manufacturer in Ohio - they have to get FDA approval first. That’s where it gets complicated.
They can submit a Prior Approval Supplement (PAS), which takes an average of 22 months to get approved. Or they can use a Change Being Effected in 30 Days (CBE30) if the change is minor. Either way, the original NDA holder is still legally responsible for every pill that leaves the plant.
And starting January 1, 2024, the FDA requires companies to disclose whether the authorized generic is made in the same facility as the brand-name drug. That transparency was pushed after the Government Accountability Office flagged supply chain risks in 2022.

What’s Next? The Big One: Humira
The biggest authorized generic story coming isn’t about a small blood pressure pill. It’s about Humira.
Humira, made by AbbVie, is the best-selling drug in U.S. history - over $20 billion in annual sales at its peak. Its patents are expiring in 2025. And AbbVie has already set up a subsidiary called Soliris Generics to produce the authorized generic version.
That means when the first true generic versions of Humira hit the market, they’ll be competing against an authorized generic made by AbbVie itself - same formulation, same manufacturing site, same quality controls. It’s a masterclass in market control.
Industry analysts predict that by 2025, 15-20% more authorized generics will be made through subsidiaries like this. With over $127 billion in brand-name drugs set to lose patent protection in the next five years, this model is only going to grow.
What This Means for You
When you’re prescribed a generic, ask your pharmacist: "Is this an authorized generic?" You might be getting the exact same drug your doctor prescribed - just cheaper.
And if you’re paying out of pocket, you might save even more. Authorized generics often cost less than other generics because they’re made at scale by the original manufacturer. No middlemen. No extra markups. Just the same pill, with a simpler label.
It’s not about branding. It’s about access. And sometimes, the best way to make a drug affordable is to let the company that made it - the one who knows it best - sell it at a lower price.
So next time you see a generic on the shelf, don’t assume it’s a knockoff. It might be the original - just wearing a different name tag.
Are authorized generics the same as regular generics?
Yes and no. Authorized generics contain the exact same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They’re made using the same formula and manufacturing process - often in the same facility. Regular generics are approved through a separate Abbreviated New Drug Application (ANDA) and may be made in different factories, sometimes with slightly different inactive ingredients or manufacturing methods. But both are required to be bioequivalent.
Can authorized generics be cheaper than regular generics?
Yes. Because authorized generics are made by the original brand company at scale, they often undercut even other generics in price. Since they don’t need to recoup R&D costs or build new manufacturing lines, their pricing is leaner. In some cases, the authorized generic is the lowest-priced version available - even lower than the first generic to enter the market.
Why would a brand company sell its own drug as a generic?
It’s a business strategy. When a patent is about to expire, brand companies face a sudden drop in sales as multiple generics flood the market. By launching their own authorized generic, they keep control over production, pricing, and supply. They capture market share before competitors can, and avoid the price collapse that often follows patent expiry. It’s not about altruism - it’s about staying profitable in a changing market.
Are authorized generics safer or more reliable than regular generics?
They’re not inherently safer - all FDA-approved generics must meet the same quality standards. But because authorized generics are made in the same facility as the brand-name drug, using the same processes and controls, they often have tighter batch consistency. The FDA’s inspection data shows higher compliance rates for authorized generic facilities (98.7%) compared to traditional generic plants (96.2%). For patients sensitive to small variations, this can matter.
How can I tell if my generic is an authorized generic?
Ask your pharmacist. They can check the drug’s label and manufacturer code. Authorized generics often list the brand company’s name on the label or packaging - like "Made by Pfizer" or "Distributed by Greenstone LLC." You can also search the FDA’s Orange Book database for the brand name and see if any authorized generic versions are listed under the same NDA number.
Do authorized generics delay real generic competition?
Some experts say yes. By offering a low-priced version early, brand companies can reduce the financial incentive for independent generic manufacturers to enter the market. This can delay the full wave of competition that usually drives prices down further. Critics argue this keeps overall drug prices higher than they’d be with pure generic competition. The FDA acknowledges the debate but maintains that authorized generics still provide affordable access and meet the same quality standards as all other generics.

Manoj Kumar Billigunta
January 18, 2026 AT 20:36Interesting read. I never realized that the generic I get at the pharmacy might be the exact same pill as the brand-name version. My pharmacist never mentioned it, but now I’m going to ask next time. It makes sense-same factory, same quality, lower price. Why wouldn’t you choose that?
sagar sanadi
January 19, 2026 AT 03:43Oh sure, let’s just trust Big Pharma to give us cheap versions of their own drugs. Next they’ll tell us the moon landing was real. They’re not doing this to help you-they’re locking you into their ecosystem before the real generics even show up. It’s a trap.
Art Gar
January 20, 2026 AT 09:17While the article presents a technically accurate overview of authorized generics, it fails to adequately address the ethical implications of market manipulation under the guise of patient access. The Hatch-Waxman Act was never intended to facilitate monopolistic continuation via subsidiary-driven price suppression.
Edith Brederode
January 21, 2026 AT 08:05This is so eye-opening! 😮 I’ve been taking a generic for years and never knew it might be the exact same pill as my doctor’s original prescription. I’m going to ask my pharmacist tomorrow-thank you for sharing this! 💡
Arlene Mathison
January 21, 2026 AT 13:46Wait-so if I’m paying less for a generic, but it’s made by the same company that made the brand, that means I’m getting the same quality without the marketing hype? That’s a win. I’m telling my mom about this. She’s always paranoid about generics. This is the kind of info that actually saves people money and stress.
Carolyn Rose Meszaros
January 22, 2026 AT 23:26Okay but can we talk about how wild it is that the FDA is now requiring disclosure of where it’s made?? 🤯 Like, we’re finally getting transparency. And Humira’s gonna be the biggest test case ever. I’m not even mad anymore. This is kinda brilliant.
Greg Robertson
January 23, 2026 AT 08:49My cousin works at a pharma plant in Indiana-she told me they switch labels on the same line for authorized generics. No retooling, no new batches. Just a new box. It’s kind of wild when you think about it. The whole thing’s just a branding game.
Jacob Cathro
January 23, 2026 AT 15:09so like… the brand companies are just… faking competition?? like they make the generic themselves and then pretend like they’re not the bad guys?? lmao. also why is everyone so chill about this? this is literally how monopoly capitalism works. 🤡
Paul Barnes
January 24, 2026 AT 23:27It is inaccurate to suggest that authorized generics are inherently superior to traditional generics. Both are required to meet bioequivalence standards under the FDA’s ANDA and NDA frameworks. The distinction lies in regulatory pathway, not clinical outcome.
pragya mishra
January 25, 2026 AT 09:04Why are you all so naive? If this is true, then why don’t pharmacies tell us? Because they get kickbacks from brand companies. You think they care about your savings? They care about their margins. This whole thing is a scam. Ask your doctor if they’re paid to push these.
Andy Thompson
January 25, 2026 AT 14:33USA only gets this stuff because we pay the world for pharma R&D. China and India make the real generics. We’re getting the premium version at discount prices-so stop complaining. If you want cheap drugs, go buy from overseas. But don’t cry when your pills come from a factory with no FDA oversight.
Thomas Varner
January 26, 2026 AT 03:59...And yet, the FDA’s inspection data shows a 98.7% compliance rate for authorized generic facilities... which is higher than the 96.2% for traditional generic plants... which suggests, statistically, that the manufacturing environment matters... even if the active ingredient is identical... which means, perhaps, that the inactive ingredients, or the process controls, or the humidity levels in the drying chamber... actually do affect consistency... which is why some patients report differences... even when the FDA says they shouldn’t...
clifford hoang
January 26, 2026 AT 04:00Think about it: the same molecules, the same machines, the same hands… but the label changes everything. It’s like a Shakespearean tragedy-Hamlet, but called ‘Prince of Denmark’. The soul is unchanged, but the world sees it as lesser. We’ve commodified identity. We’ve turned medicine into a branding exercise. Who are we, really, if not the names we wear?
Crystal August
January 27, 2026 AT 03:50So let me get this straight-companies are allowed to make their own generic versions to block competition? That’s not innovation. That’s legal sabotage. And you’re all acting like this is some noble act of affordability? No. It’s corporate control dressed up in healthcare clothing.
Nadia Watson
January 27, 2026 AT 21:28This is fascinating, though I must admit, I misread 'Greenstone LLC' as 'Green Stone' at first and imagined a literal rock factory. 😅 I’ve always trusted generics, but now I’ll ask for the authorized ones. Thank you for the clarity. The transparency requirement starting in 2024 feels like a small but meaningful step forward.